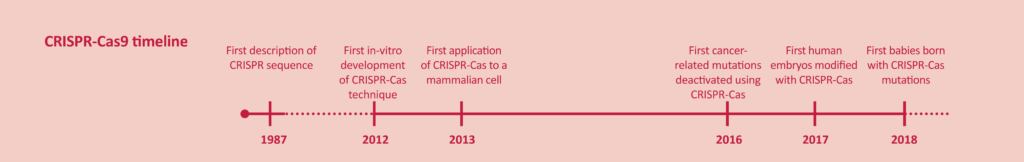
CRISPR-Cas refers to a promising gene modification technique. The revolutionary potential of CRISPR technology lies in its simplicity, speed, precision and low cost. Genetic material is snipped at a specific site, allowing fragments of genetic material to be inserted or removed. The risk of side-effects is minimised by the high precision of CRISPR. The technique has wide applications for plants, animals and humans. Researchers anticipate benefits including repairing genes or combating infectious diseases.
The technology has experienced exponential development in recent years. Treatments are already being developed on the basis of the gene modification of body cells. In previous editions we described experiments to treat disorders such as β-thalassaemia and cancer [1,2]. Experiments with germline cells, which transmit genetic material to the progeny, have been long in coming. Gene modification in such cells is a sensitive issue, because the modified hereditary information would be passed on to offspring. Any side-effects or long-term consequences of CRISPR are still unclear [2,3]. However, in November 2018 the world was shocked after an announcement by the Chinese scientist, He Jiankui, that he had succeeded in ‘creating’ the first CRISPR-modified human babies[3-5].
CRISPR technology has enabled a variety of new therapies and products. New ethical and medical dilemmas have consequently been raised, illustrated by ‘biohackers’ who inject themselves with CRISPR to spark extra muscle growth [6]. Sjoerd Repping, who heads the Centre for Reproductive Medicine at the Amsterdam University Medical Centre, has argued: “The technique is here. Full stop. It will never go away. It is going to happen – in fact, it has already happened. But we do need to ask ourselves how it can be done safely, and above all we have to determine for which people it is advisable.” [7]. Applications of gene editing could have serious ramifications for public health. Inserted genetic traits can spread quickly, and irreversibly, throughout the population of an organism, and from there to the whole living environment [8,9].
Genetic modification of microorganisms has potential for infectious disease control. An example is the recently modified mosquito that can no longer transmit the malaria parasite [10]. Yet unintended new variants could also result.
Therapeutic developments have occurred of late in the field of CRISPR technology, including interventions for dementia, haemophilia and Duchenne muscular dystrophy [8-10]. Researchers at the Delft University of Technology have also devised a method to make the technique more predictable and stable [11,12] Numerous studies are in progress with varied research focuses, including oncology, viral infections and eye diseases [13]. In some cases, commercial CRISPR services for the health care sector are being developed [1,14-16].
The developments in CRISPR technology reveal its tremendous potential. High-precision modifications in the genetic material of human beings or of pathogenic organisms have brought the cure of many diseases into sight. At the same time, the recent developments are also impressing upon us the importance of working cautiously within an ethical and regulatory framework. A possible problem lies in international differences in notions about genetic disorders, as well as in legal and regulatory stipulations [17,18]. CRISPR has the potential to further personalise health care, and it will make significant contributions to precision medicine. The first CRISPR medical applications are already finding their way to the health sector. It is our obligation to deal with them properly.
References
- CrisprTX.com 2019. Available from: www.crisprtx.com. Retrieved 19-01-19
- Reardon S. First CRISPR clinical trial gets green lightfrom US panel. Nature News. 2016
- Cyranoski D. CRISPR-baby scientist fails to satisfy critics. Nature. 2018;564(7734):13
- Wang C, Chinese Academy of Medical Sciences B, China, Zhai X, Chinese Academy of Medical Sciences B, China, Zhang X, Chinese Academy of Medical Sciences B, China, et al. Gene-edited babies: Chinese Academy of Medical Sciences’ response and action. The Lancet. 2018;393(10166):25-6
- CRISPR-Cas9: a world first? – The Lancet. December 2018
- Zhang S. A Biohacker Regrets Publicly Injecting Himself With CRISPR. February 2018
- NOS. Een mens naar wens: zo ver gaan we al met knutselen aan dna: nosop3; 2018. Available from: https://nos.nl/op3/artikel/2261686-een-mens-naar-wens-zover-gaan-we-al-met-knutselen-aan-dna.html. Retrieved on 14-01-19Meacham JM, Durvasula K, Degertekin FL, Fedorov AG. Enhanced intracellular delivery via coordinated acoustically driven shear mechanoporation and electrophoretic insertion. Scientific Reports. 2018;8(1):3727Maron DF. CRISPR Gene Editing Shows Promise for Treating a Fatal Muscle Disease – Scientific American. 2018
- Meacham JM, Durvasula K, Degertekin FL, Fedorov AG. Enhanced intracellular delivery via coordinated acoustically driven shear mechanoporation and electrophoretic insertion. Scientific Reports. 2018;8(1):3727
- Maron DF. CRISPR Gene Editing Shows Promise for Treating a Fatal Muscle Disease – Scientific American. 2018
- Konermann S, Lotfy P, Brideau NJ, Oki J, Shokhirev MN, Hsu PD. Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell. 2018;173(3):665-76. e14
- Grand View Research. Regenerative Medicine Market Size | Industry Trends Report, 2018-2025: GrandViewInc; 2018 Available from: https://www.grandviewresearch.com/industry-analysis/regenerative-medicine-market. Retrieved 18-01-19
- Gorter de Vries AR, Couwenberg LGF, van den Broek M, de la Torre Cortés P, ter Horst J, Pronk JT, et al. Allele-specific genome editing using CRISPR–Cas9 is associated with loss of heterozygosity in diploid yeast.Nucleic Acids Research. 2018:gky1216-gy
- Mollanoori H, Teimourian S. Therapeutic applications of CRISPR/Cas9 system in gene therapy. Biotechnology Letters. 2018;40(6):907-14
- CRISPR Products and Services – US 2019. Available from: https://www.thermofisher.com/us/en/home/life-science/genome-editing/geneart-crispr.html. Retrieved on 12-01-19
- Nanalyze. 6 CRISPR Applications from Healthcare Startups. August 2018
- Beam Therapeutics. Available from: https://beamtx.com/. Retrieved on 17-01-19
- Nature. How to respond to CRISPR babies. Nature. 2018;564(7734):5
- Sim EU-H, Ting S-H. Patterns and Determinants of Attitudes towards Genetic Risk of Cancer: Case Study in a Malaysian Public University. BioMed Research International. 2018;2018:7